Can the Existence of Isotopes Explain the Difference in Properties
How can isotopes be used in medicine be specific. Buckminsterfullerene C 60 is another allotrope of carbon.

Solved Can Ihe Existence Of Isotopes Explain The Difference Diamond And Graphite Explain Properties Between Propose Explanation For The Difference In Properties Between Diamond And Graphite Use Grammatically Correct English Sentences 18 Oz
Give two examples of each that exist in the human body and explain their function.
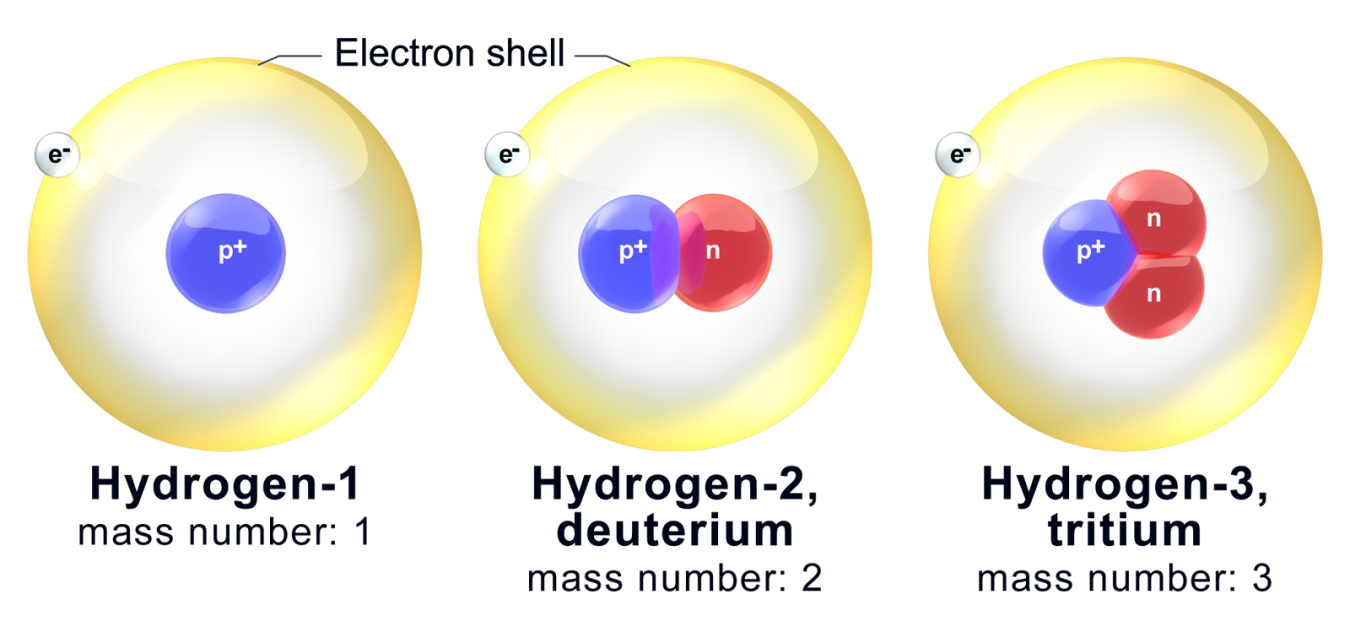
. This is because the number of electrons determines chemical properties and all three isotopes have one electron in their atoms. Their chemical property is different because there is a difference in the number of electrons. The difference in mass is often expressed as energy by using Albert Einsteins relativity equation in the form E Δmc 2.
The example of two Isotopes and Isobars is iron and nickel. Buckminsterfullerene C-60 is another allotrope of carbon. Isotopes can be considered as variants of the same element the only difference is in their number of protons and number of neutrons which exists in the nuclei of atoms.
Lets use carbon as an example. The number of neutrons and the number of protons together makes the mass number of an element so the mass number of isotopes is different. Differences in the properties of isotopes can be attributed to either of two causes.
In order to calculate this quantity the natural abundance and. Propose an explanation for the difference in properties between diamond and graphite. Isotopes are either stable or radioactive.
O 2 and O 3 ozone are allotropes of oxygen. O2 and O3 ozone are allotropes of oxygen. O 2 and O 3 ozone are allotropes of oxygen.
The difference Δm between the actual mass of the assembled isotope and the masses of the particles gives a measure of the stability of the isotope. Buckminsterfullerene Coo is another allotrope of carbon. Up to 24 cash back Can the existence of isotopes explain the difference in properties between diamond and graphite.
It has the same atomic mass but different atomic no. Explain the difference between the MASS NUMBER and the ATOMIC NUMBER of a nuclide. This is because an additional number of neutrons compensates the difference in the number of nucleons.
Radioactive isotopes are called. In order to calculate this quantity the natural abundance and. Generally the chemical properties of isotopes of any element are almost identical.
Therefore the periodic table lists a weighted average atomic mass for each element. Diamond is still a better conductor of heat than the isoptically pure silicon materials. Therefore the periodic table lists a weighted average atomic mass for each element.
Chemical properties of different isotopes are almost similar. Propose an explanation for the difference in properties between diamond and graphite. Answered 4 years ago Author has 11K answers and 13M answer views.
Recall that all isotopes of an element have the same physical and chemical properties with the exception of atomic mass and for unstable isotopes radioactivity. Elements rarely occur as only one isotope. What are isotopes and how are isotopes distinguished from one another.
The larger and more negative the value of Δm the greater the stability of the isotope. Allotropy describes the arrangement of atoms in an element causing each form of the element to have different chemical properties. All three isotopes of hydrogen have identical chemical properties.
The exception to this case is the isotopes of hydrogen because the numbers of neutrons have a major effect on the size of the nucleus of a hydrogen atom. Consider the case of two things that appear to be the same in color and physical appearance in a way that we cannot distinguish between them. Scientists usually refer to the former as isotope effects and to the latter by a variety of more specialized names.
The physical properties of any isotope are largely. Up to 24 cash back Recall that all isotopes of an element have the same physical and chemical properties with the exception of atomic mass and for unstable isotopes radioactivity. Mass and temperature are all important aspects of atomic properties.
Use grammatically correct English sentences. Propose an explanation for the difference in properties between diamond and graphite. It has little difference in terms of chemical composition.
Describe in detail one example of how an isotope is used therapeutically and describe another example of how an isotope is used. There are three isotopes of carbon found in nature carbon-12. Can the existence of isotopes explain the difference in properties between diamond and graphite.
This example relates to the idea of isotopes. Isotopes have the same chemical and physical properties so that could not explain the difference. Can the existence of isotopes explain the difference in properties between diamond and graphite.
Physical properties like mass melting or boiling point density and freezing point are different and depends on the mass of each isotope. When we compare the weights of these two then we can notice a difference. Differences in mass or differences in nuclear structure.
When it comes to physical properties of isotopes including mass melting or boiling point density and freezing point they are all different. Isotopes of an element have different physical properties because they have different mass numbers. The physical properties of isotopes in a particular element vary from each other.
Isotopy defines a property of chemical elements whereby atoms of the same element have the same proton number but different mass number. Isotopes are elements that have similar atomic numbers but different mass numbers. Up to 24 cash back Recall that all isotopes of an element have the same physical and chemical properties with the exception of atomic mass and for unstable isotopes radioactivity.
Using a weighted average atomic mass you can account for the less common isotopes. 2- all atoms of an element are identical in size mass and. Rather they exist as mixtures of different isotopes of various masses.
The chemical properties are almost identical as different isotopes show almost identical chemical behaviours. Both have the same mass number which is 58 whereas the. In order to calculate this quantity the natural abundance and.
Hence the difference in properties is due to the existence of different allotropes of. As far as the chemical property of isotopes is concerned the properties of elements of nearly alike or identical composition. Therefore the periodic table lists a weighted average atomic mass for each element.
Isotopes of an element share the same number of protons but have different numbers of neutrons. There are isotopically refined silicon materials that have improved thermal conductivity and they have specific uses in power electronics but they dont have the same properties as diamond or graphite. Up to 24 cash back The difference in properties is because red and white phosphorus are two different allotropes of phospho- rus.

Solved B Can The Existence Of Isotopes Explain The Chegg Com
No comments for "Can the Existence of Isotopes Explain the Difference in Properties"
Post a Comment